No results were found for the filter!
NEW
 pS355-β2 (phospho-β2-Adrenoceptor Antibody)
pS355-β2 (phospho-β2-Adrenoceptor Antibody) Serine355 (S355) is a major phosphorylation site of the β2 adrenoceptor. The pS355-β2 antibody detects phosphorylation in response to high- and low-efficacy agonists but not after PKC activation. S355 phosphorylation is primarily...
$ 375.00 *
NEW
 pS356-β2 (phospho-β2-Adrenoceptor Antibody)
pS356-β2 (phospho-β2-Adrenoceptor Antibody) Serine356 (S356) is a major phosphorylation site of the β2 adrenoceptor. The pS356-β2 antibody detects phosphorylation in response to high- and low-efficacy agonists but not after PKC activation. S356 phosphorylation is primarily...
$ 375.00 *
NEW
 pT360-β2 (phospho-β2-Adrenoceptor Antibody)
pT360-β2 (phospho-β2-Adrenoceptor Antibody) Threonine360 (T360) is a major phosphorylation site of the β2 adrenoceptor. The pT360-β2 antibody detects phosphorylation in response to high- and low-efficacy agonists but not after PKC activation. T360 phosphorylation is primarily...
$ 350.00 *
NEW
 pS351/pS353-S1P1 (phospho-Sphingosine...
pS351/pS353-S1P1 (phospho-Sphingosine... Serine351/Serine353 (S351/S353) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 1 (S1P1). The pS351/pS353-S1P1 antibody detects phosphorylation in response to agonists. S351/S353 phosphorylation is likely to be...
$ 375.00 *
NEW
 pS330/pS331-S1P2 (phospho-Sphingosine...
pS330/pS331-S1P2 (phospho-Sphingosine... Serine330/Serine331 (S330/S331) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 2 (S1P2). The pS330/pS331-S1P2 antibody detects phosphorylation in response to agonists. S330/S331 phosphorylation is likely to be...
$ 375.00 *
NEW
 pS366/pS367-S1P5 (phospho-Sphingosine...
pS366/pS367-S1P5 (phospho-Sphingosine... Serine366/Ser367 (S366/S367) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 5 (S1P5). The pS366/pS367-S1P5 antibody detects phosphorylation in response to agonists. S366/S367 phosphorylation is likely to be...
$ 375.00 *
NEW
 pS353/pS355-S1P1 (phospho-Sphingosine...
pS353/pS355-S1P1 (phospho-Sphingosine... Serine353/Serine355 (S353/S355) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 1 (S1P1). The pS353/pS355-S1P1 antibody detects phosphorylation in response to agonists. S353/S355 phosphorylation is likely to be...
$ 375.00 *
NEW
 pS358/pS359-S1P1 (phospho-Sphingosine...
pS358/pS359-S1P1 (phospho-Sphingosine... Serine358/Serine359 (S358/S359) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 1 (S1P1). The pS358/pS359-S1P1 antibody detects phosphorylation in response to agonists. S358/S359 phosphorylation is likely to be...
$ 375.00 *
NEW
 pT342/pS343/pT345-S1P2 (phospho-Sphingosine...
pT342/pS343/pT345-S1P2 (phospho-Sphingosine... Threonine342Serine343/Threonine345 (T342/S343/T345) is major phosphorylation site of the Sphingosine 1-Phosphate Receptor 2 (S1P2). The pT342/pS343/pT345-S1P2 antibody detects phosphorylation in response to agonists. T342/S343/T345...
$ 375.00 *
NEW
 pS383/pS384-PAR2 (phospho-Proteinase-Activated...
pS383/pS384-PAR2 (phospho-Proteinase-Activated... Serine383/Serine384 (S383/S384) is major phosphorylation site of the Proteinase-Activated Receptor 2 (PAR2). The pS383/pS384-PAR2 antibody detects phosphorylation in response to agonists. S383/S384 phosphorylation is likely to be...
$ 375.00 *
NEW
 pT338-HCA2 (phospho-Hydroxycarboxylic Acid...
pT338-HCA2 (phospho-Hydroxycarboxylic Acid... Threonine338 (T338) is major phosphorylation site of the Hydroxycarboxylic Acid Receptor 2 (HCA2). The pT338-HCA2 antibody detects phosphorylation in response to agonists. T338 phosphorylation is likely to be involved in efficient ligand...
$ 375.00 *
NEW
 pT347-FFA4 (phospho-FFA4 Antibody)
pT347-FFA4 (phospho-FFA4 Antibody) Threonine347 (T347) is major phosphorylation site of the FFA Receptor 4 (FFA4). The pT347-FFA4 antibody detects phosphorylation in response to agonists. T347 phosphorylation is likely to be involved in efficient ligand sequestration by...
$ 375.00 *
NEW
 pT328/pS329-FFA3 (phospho-FFA3 Antibody)
pT328/pS329-FFA3 (phospho-FFA3 Antibody) Threonine328/Serine329 (T328/S329) is major phosphorylation site of the FFA Receptor 3 (FFA3). The pT328/pS329-FFA3 antibody detects phosphorylation in response to agonists. T328/S329 phosphorylation is likely to be involved in efficient...
$ 375.00 *
NEW
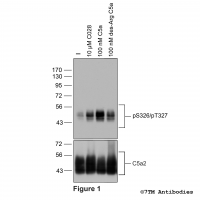 pS326/pT327-C5a2 (phospho-Complement C5a...
pS326/pT327-C5a2 (phospho-Complement C5a... Serine326/Threonine327 (S326/T327) is major phosphorylation site of the Complement Peptide Receptor 2 (C5a2). The pS326/pT327-C5a2 antibody detects phosphorylation in response to agonists. S326/S327 phosphorylation is likely to be...
$ 375.00 *
NEW
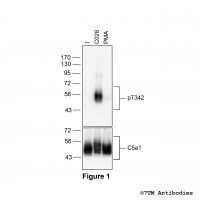 pT342-C5a1 (phospho-Complement C5a Receptor 1...
pT342-C5a1 (phospho-Complement C5a Receptor 1... Threonine342 (T342) is a major phosphorylation site of the C5a1 receptor. The pT342-C5a1 antibody detects phosphorylation in response to high- and low-efficacy agonists but not after PKC activation. T342 phosphorylation is a key...
$ 375.00 *
NEW
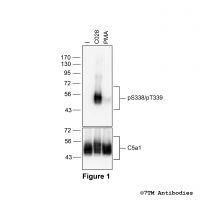 pS338/pT339-C5a1 (phospho-Complement C5a...
pS338/pT339-C5a1 (phospho-Complement C5a... Serine338/Threonine339 (S338/T339) is a major phosphorylation site of the C5a1 receptor. The pS338/pT339-C5a1 antibody detects phosphorylation in response to high- and low-efficacy agonists but not after PKC activation. S338/T339...
$ 375.00 *
Recently viewed