Gastric Inhibitory Polypeptide Receptor Antibodies
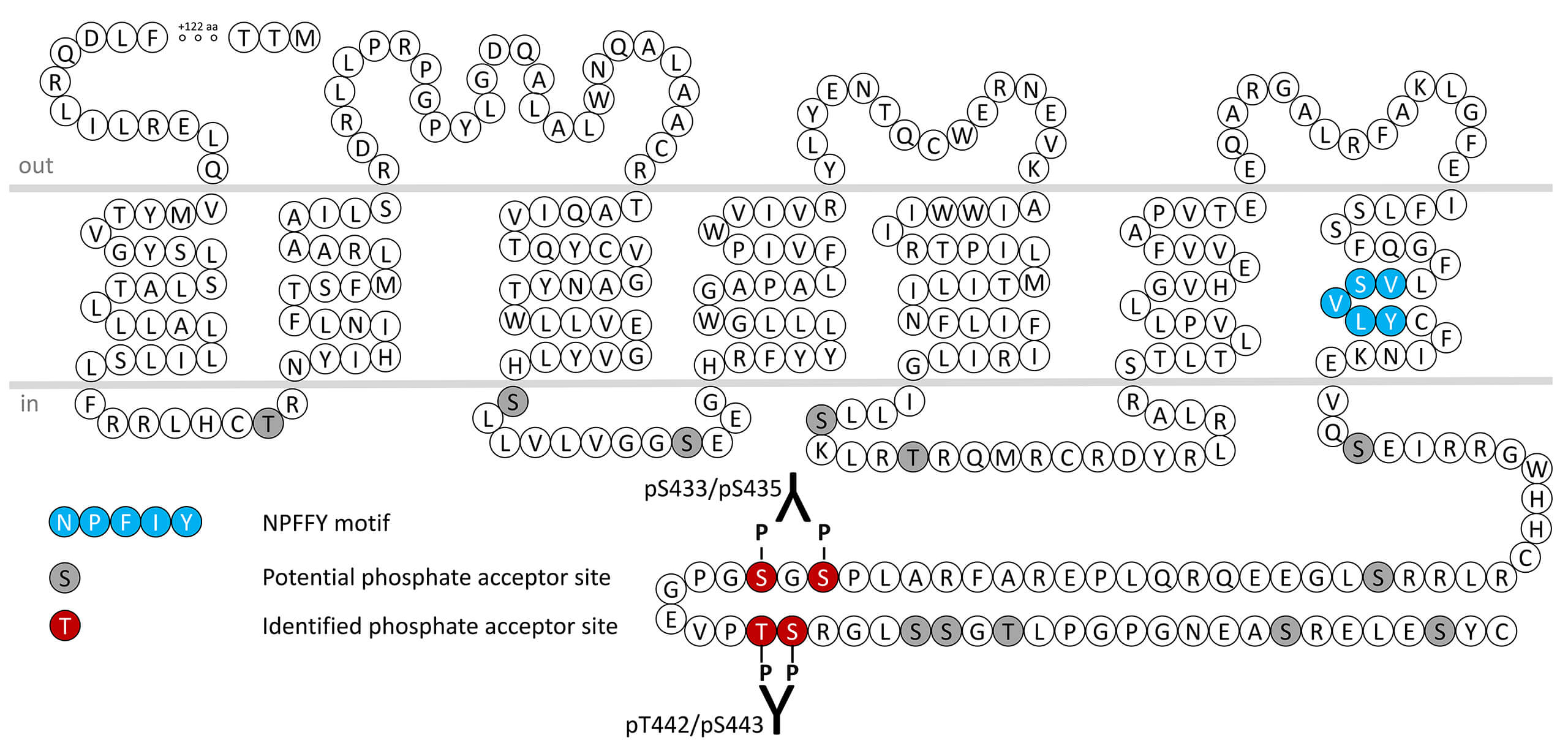
Gastric inhibitory polypeptide (GIP), also known as glucose-dependent inhibitory polypeptide is synthesised in and secreted from enteroendocrine K-cells in the duodenum and jejunum. Although originally identified as an inhibitor of gastric acid secretion, the best known action of GIP is the potentiation of glucose-dependent insulin secretion via GIP receptors expressed on islet β-cells. The actions of GIP are modulated by the physiological degradation of the peptide via N-terminal cleavage by the aminopeptidase dipeptidyl peptidase IV. GIP receptors preferentially couple through Gs protein to stimulate adenylate cyclase. GIP receptor desensitization, β-arrestin recruitment and internalization are regulated by phosphorylation of carboxyl-terminal serine433/serine435 (pS433/pS435-GIPR) and threonine442/serine443 (pT442/pS443-GIPR). This nomenclature refers to the human GIPR. For more information on GIPR pharmacology please refer to the IUPHAR database. For further reading refer to:
Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003 Mar;55(1):167-94. doi: 10.1124/pr.55.1.6. PMID: 12615957.
Bataille D, Chan SL, Delagrange P, Drucker DJ, Göke B, Hills R, Mayo KE, Miller LJ, Salvatori R, Thorens B. Glucagon receptor family in GtoPdb v.2023.1. IUPHAR/BPS Guide to Pharmacology CITE. 2023; 2023(1).
 pT442/pS443-GIPR (phospho-Gastric Inhibitory...
pT442/pS443-GIPR (phospho-Gastric Inhibitory...  pS433/pS435-GIPR (phospho-Gastric Inhibitory...
pS433/pS435-GIPR (phospho-Gastric Inhibitory...