Formylpeptide Receptor 2 Antibodies
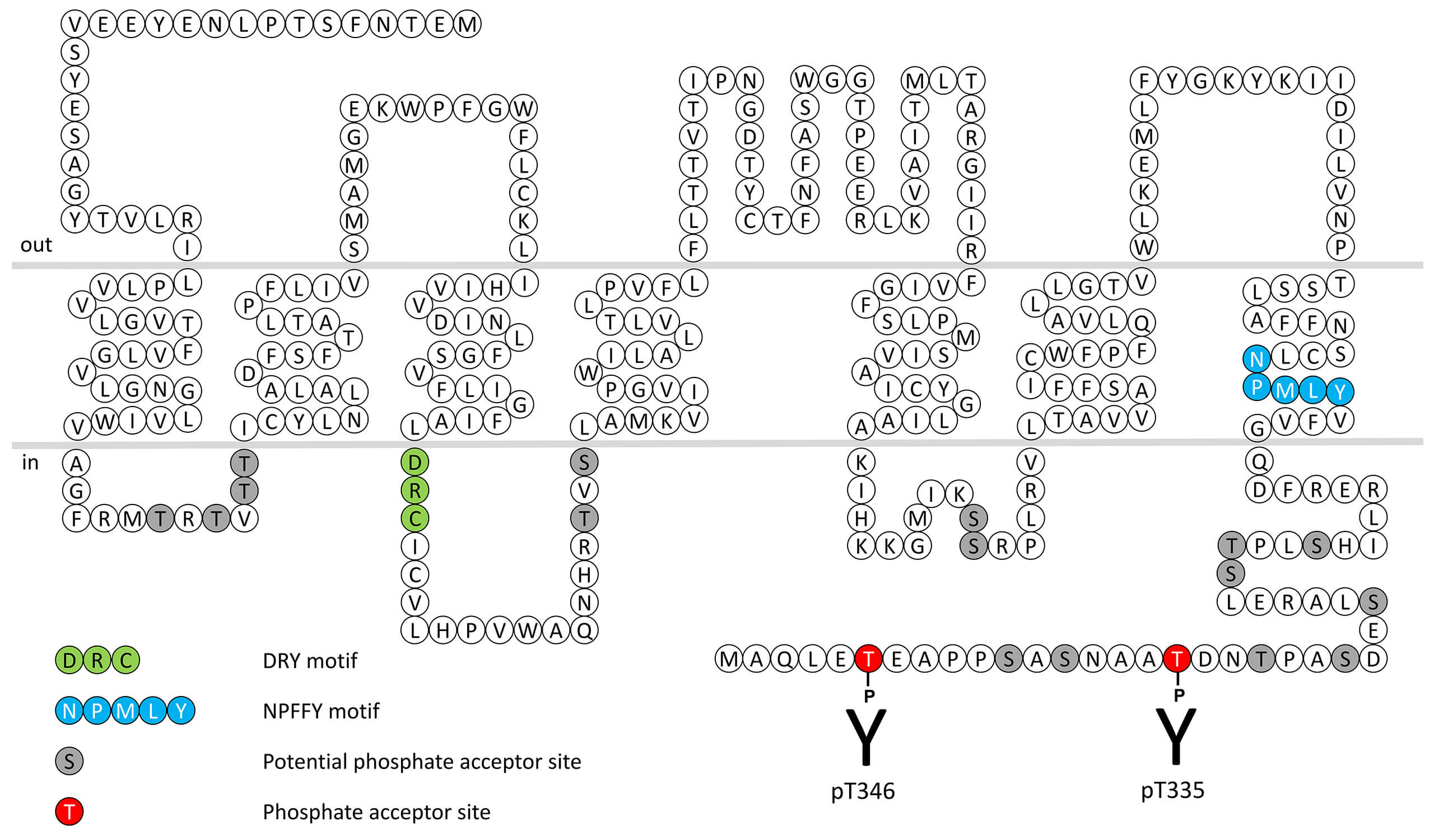
The primary function of FPR2 is to interact with a variety of ligands with different structures including lipid mediators such as lipoxin A4. The formylpeptide receptors respond to exogenous ligands such as the bacterial product fMet-Leu-Phe (fMLP). Bacterial protein synthesis is initiated with an N-Formylmethionine (fMet) at the amino terminus. This feature of prokaryotic protein synthesis is used by mammalian immune cells to recognize the invading bacteria. These bacterial peptides are able to induce chemotaxis, degranulation and superoxide production, which are important bactericidal functions. The human genome contains 3 genes coding for a subfamily of three formylpeptide receptors (FPRs). FPRs interact with a diverse collection of exogenous and endogenous ligands including lipoxin A4, annexin I, cathepsin G, amyloid β42 and spinorphin and are among the most promiscuous GPCRs known to date. FPR2 receptor desensitization, β-arrestin recruitment and internalization are regulated by phosphorylation of carboxyl-terminal threonine335 (pT335-FPR2) and threonine346 (pT346-FPR2). This nomenclature refers to the human FPR2 receptor. For more information on FPR2 pharmacology please refer to the IUPHAR database. For further reading refer to:
Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009 Jun;61(2):119-61. doi: 10.1124/pr.109.001578. Epub 2009 Jun 4. PMID: 19498085; PMCID: PMC2745437.
 pT335-FPR2 (phospho-Formylpeptide Receptor 2...
pT335-FPR2 (phospho-Formylpeptide Receptor 2...  pT346-FPR2 (phospho-Formylpeptide Receptor 2...
pT346-FPR2 (phospho-Formylpeptide Receptor 2...