V1B Vasopressin Receptor Antibodies
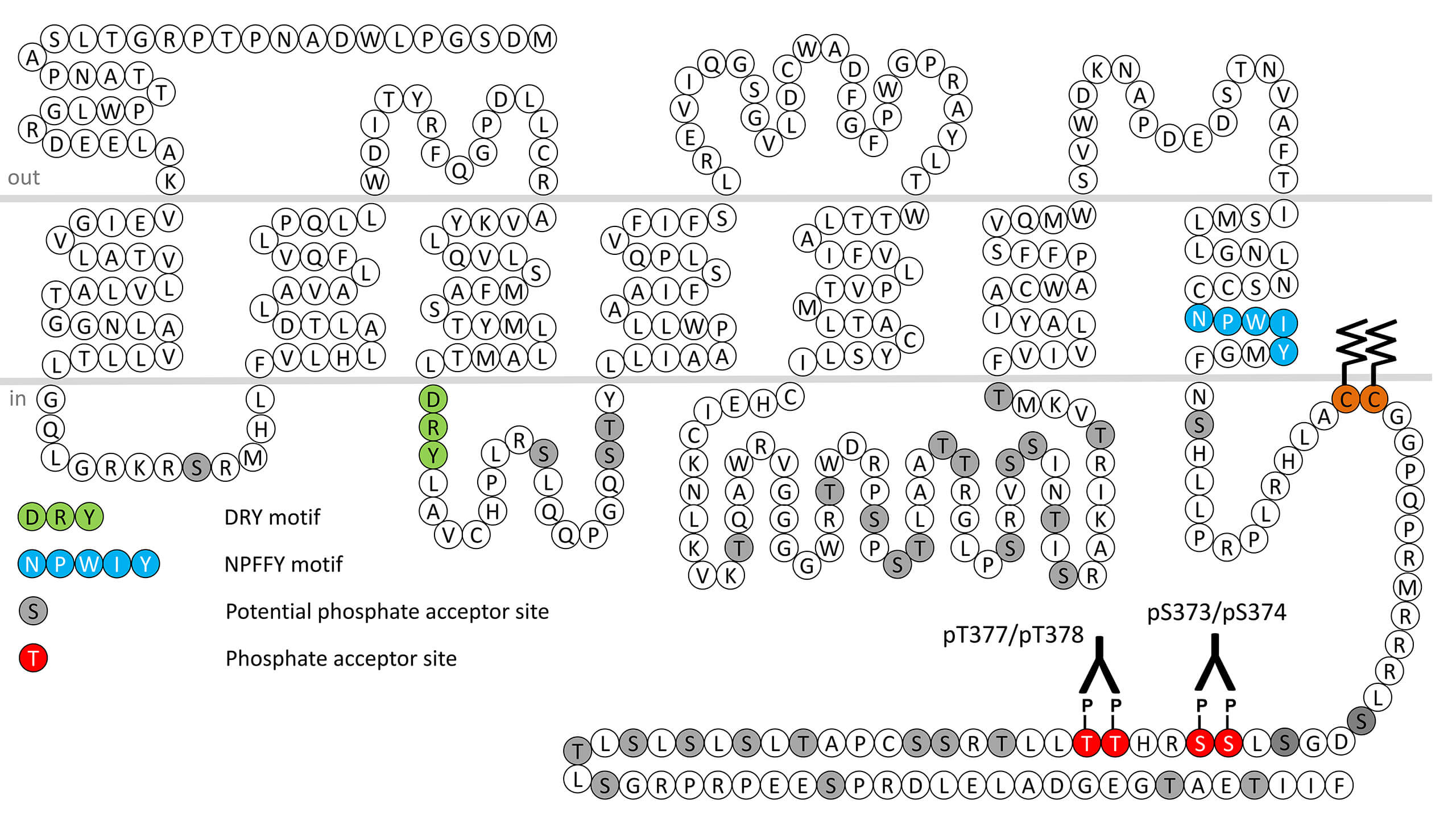
The V1B receptor is named the corticotrope AVP receptor. Like the V1A subtype, the V1B is primarily coupled to an increase in phosphatidylinositol breakdown with production of inositol triphosphate and diacylglycerol leading to a rise in cytosolic free calcium concentration and activation of proteine kinase C, respectively. Production of inositol phosphates is generated through activation of Gq protein and phospholipase C. The V1B is also coupled to Gs and activation of adenylyl cyclase, although with a much lower potency. The V1B is mostly expressed in the pituitary gland (corticotroph cells) and in beta cells of the pancreas islets, but also in multiple brain regions and other peripheral tissues, including kidney, thymus, heart, lung, spleen, uterus, adrenal gland and breast. This receptor can be distinguished from the vascular/hepatic V1A and renal V2 AVP receptors by its differential binding affinities for structural analogues of AVP. Selective V1B agonists and antagonists have been developed. The AVP receptor present at the surface of corticotroph cells is the V1B subtype. Compared with CRH, AVP itself has a weak ability of upregulating the ACTH secretion; however it markedly potentiates the effects of CRH. Altogether, CRH and AVP are characterized as upstream regulators of the HPA axis. Among the plethora of physiological processes regulated by AVP is the homeostatic control of blood glucose levels. Indeed, AVP is involved in insulin release from pancreatic beta cells and this activity is mediated via the V1B subtype. The activation of the V1B subtype of islet cells decreases blood glucose level, but AVP is also able to increase the blood glucose level by promoting the release of glucagon and by enhancing glycogenolysis in the liver. The hepatic glycogenolysis is mediated predominantly by the V1A AVP receptor. The AVP effect on insulin release is entirely lost in mice lacking the V1B subtype but was preserved in mice lacking the V1A receptor subtype. V1B receptor knockout mice also exhibit reduced basal adrenocorticotropin and corticosterone levels, an impaired rise in adrenocorticotropin in response to AVP, altered stress-induced catecholamine release and reduced aggressive behaviour. V1B receptor activity is regulated by phosphorylation of carboxyl-terminal serine373/serine374 (pS373/pS374-V1B) and threonine377/threonine378 (pT377/pT378-V1B). This nomenclature refers to the human V1B receptor. This phosphorylation motif is conserved across species and is highly homologous in mice and rats. For more information on V1B pharmacology please refer to the IUPHAR database. For further reading refer to:
Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP, Porges SW, Davis JM, Connelly JJ, Kingsbury MA. Is Oxytocin "Nature's Medicine"? Pharmacol Rev. 2020 Oct;72(4):829-861. doi: 10.1124/pr.120.019398. PMID: 32912963; PMCID: PMC7495339.
Bichet D, Bouvier M, Chini B, Gimpl G, Guillon G, Kimura T, Knepper M, Lolait S, Manning M, Mouillac B, O'Carroll AM, Serradeil-Le Gal C, Soloff M, Verbalis JG, Wheatley M, Zingg HH. Vasopressin and oxytocin receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE. 2019; 2019(4).
 pS373/pS374-V1B (phospho-Vasopressin Receptor...
pS373/pS374-V1B (phospho-Vasopressin Receptor...  pT377/pT378-V1B (phospho-Vasopressin Receptor...
pT377/pT378-V1B (phospho-Vasopressin Receptor...