No results were found for the filter!
NEW
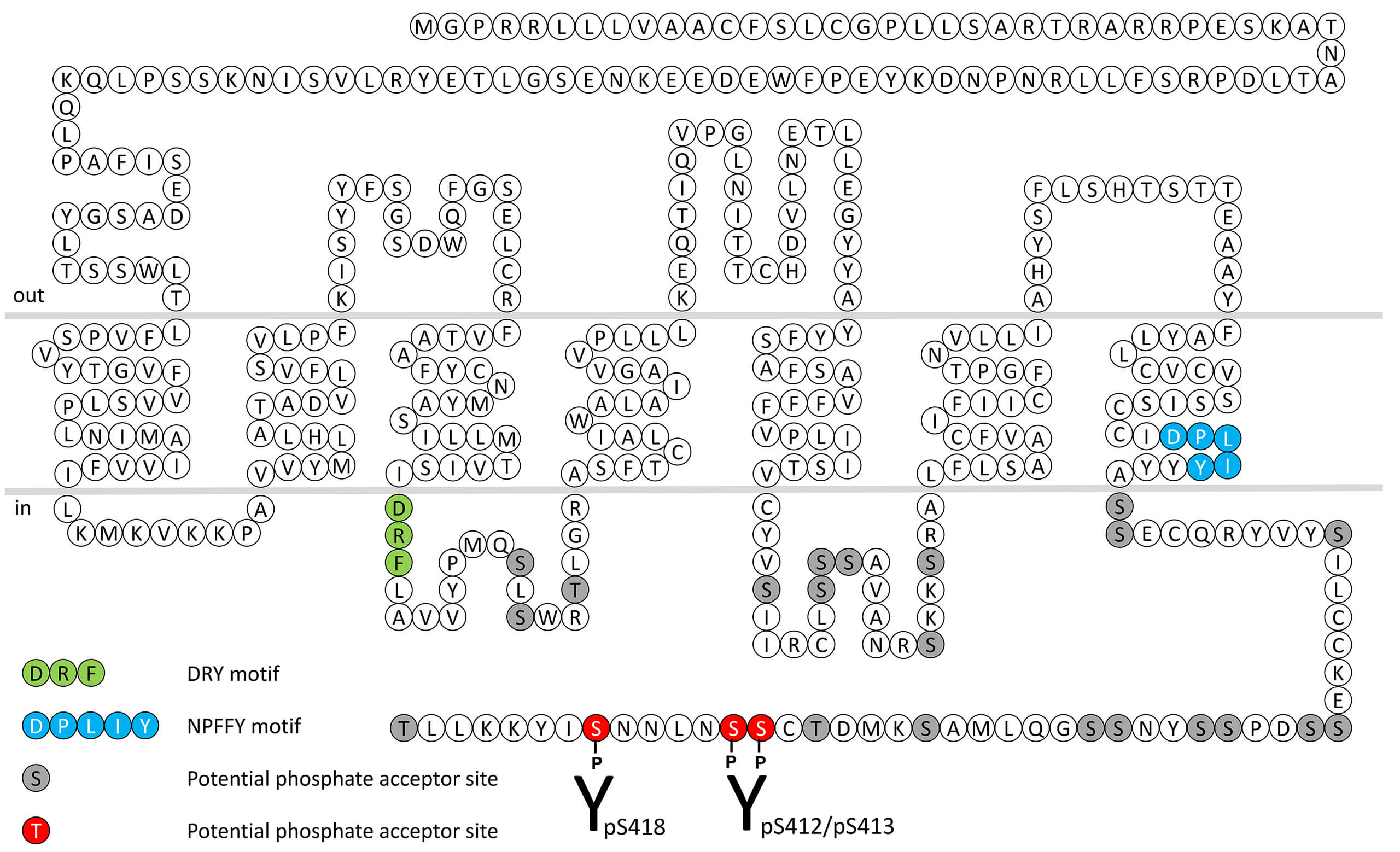
 pS412/pS413-PAR1 (phospho-Proteinase-Activated...
pS412/pS413-PAR1 (phospho-Proteinase-Activated... Serine412/Serine413 (S412/S413) is major phosphorylation site of the Proteinase-Activated Receptor 1 (PAR1). The pS412/pS413-PAR1 antibody detects phosphorylation in response to agonists. S412/S413 phosphorylation is likely to be...
$ 375.00 *
NEW
 pS418-PAR1 (phospho-Proteinase-Activated...
pS418-PAR1 (phospho-Proteinase-Activated... Serine418 (S418) is major phosphorylation site of the Proteinase-Activated Receptor 1 (PAR1). The pS418-PAR1 antibody detects phosphorylation in response to agonists. S418 phosphorylation is likely to be involved in efficient ligand...
$ 375.00 *
NEW
 PAR1 (non-phospho), Proteinase-Activated...
PAR1 (non-phospho), Proteinase-Activated... The PAR1 receptor antibody is directed against the distal end of the carboxyl-terminal tail of human Proteinase-Activated Receptor 1. It can be used to detect total PAR1 receptors in Western blots independent of phosphorylation. The PAR1...
$ 300.00 *
Recently viewed