Mas-related G-protein coupled receptor member X2 (MRGPRX2) Phosphorylation Assays
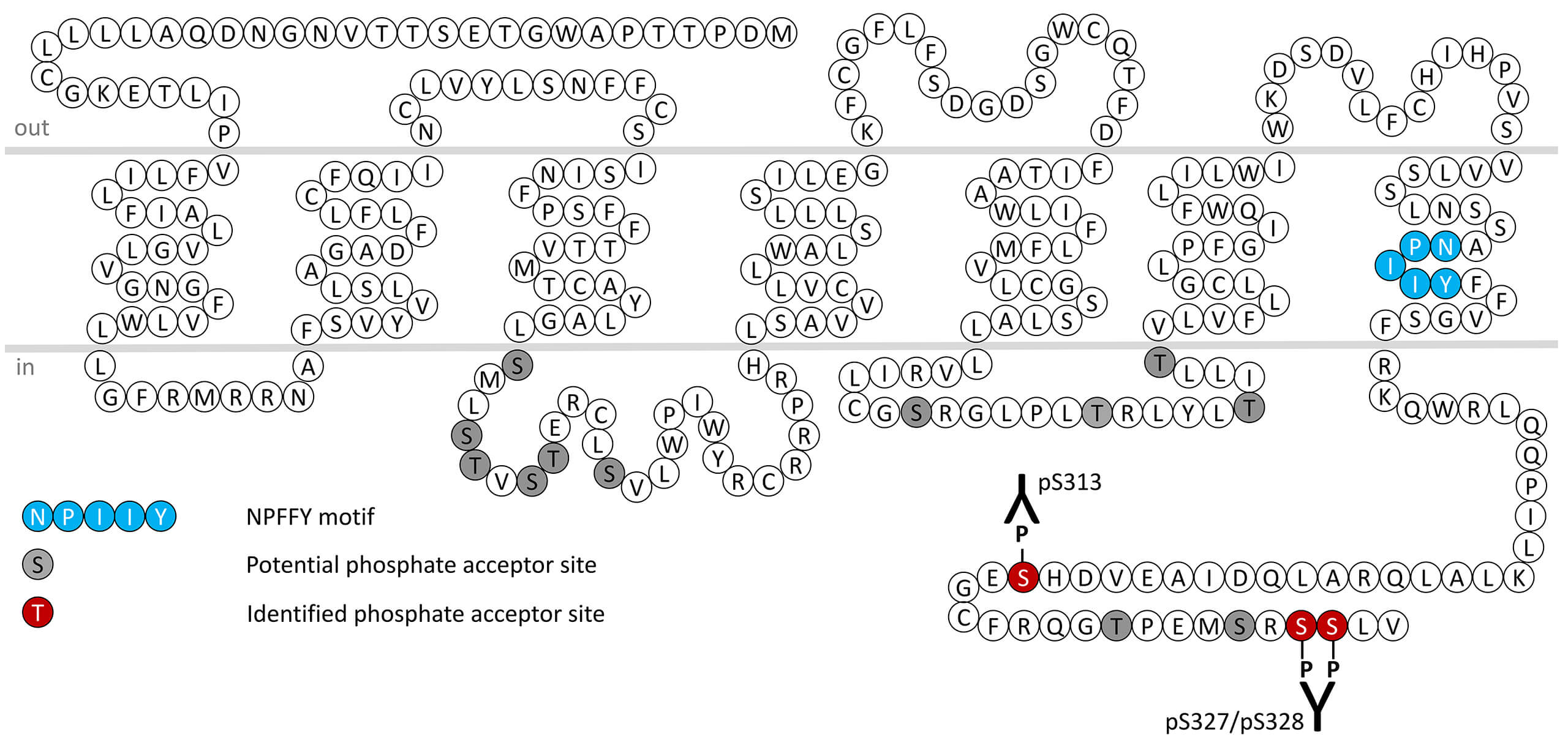
A diverse range of substances has been reported to be agonists of MRGPRX2, with cortistatin 14 the highest potency agonist in assays of calcium mobilisation also confirmed in an independent study using an arrestin recruitment assay. Compound 48/80 is used experimentally as the prototype MRGPRX2 agonist. Cortistatin and PAMP are proposed to be MRGPRX2 receptor surrogates, as both ligands have not been shown to activate MRGPRX2 in vivo. Expression of MRGPRX2 was initially reported predominantly in sensory neurons of the dorsal root ganglion. More recently expression has been detected in human mast cells. In these cells, the receptor is activated by the antimicrobial peptide LL-37, resulting in calcium mobilisation and degranulation. Of additional interest is the presence of elevated MRGPRX2 expression in skin mast cells derived from chronic urticaria sufferers, accompanied by eosinophil infiltration. Eosinophil-derived MBP (eosinophil major basic protein) and EPO (eosinophil peroxidase) have also been shown to stimulate mast cells via MRGPRX2, providing a mast cell-eosinophil cross-talk leading to disease exacerbation. It is reasoned that pharmacological antagonism of MRGPRX2 activity could represent a novel mechanism for treating IgE-independent inflammatory reactions, including anaphylaxis. Thus, MRGPRX2 is considered a molecular target for pharmacological intervention in mast cell-mediated allergic and inflammatory diseases. MRGPRX2 receptor desensitization, β-arrestin recruitment and internalization are regulated by phosphorylation of carboxyl-terminal serine313 (pS313-MRGPRX2) and and serine327/serine328 (pS327/pS328-MRGPRX2). This nomenclature refers to the human MRGPRX2 receptor. In mice, the MRG genes (encoded by MrgprB2) has been proposed to be orthologous to human MRGPRX2. For more information on MRGPRX2 pharmacology please refer to the IUPHAR database. For further reading refer to:
Alexander SP, Battey J, Benson HE, Benya RV, Bonner TI, Davenport AP, Dhanachandra Singh K, Eguchi S, Harmar A, Holliday N, Jensen RT, Karnik S, Kostenis E, Liew WC, Monaghan AE, Mpamhanga C, Neubig R, Pawson AJ, Pin JP, Sharman JL, Spedding M, Spindel E, Stoddart L, Storjohann L, Thomas WG, Tirupula K, Vanderheyden P. Class A Orphans in GtoPdb v.2023.1. IUPHAR/BPS Guide to Pharmacology CITE. 2023; 2023(1). Available from: https://doi.org/10.2218/gtopdb/F16/2023.1.
 pS327/pS328-MRGPRX2 Phosphorylation Assay Kit
pS327/pS328-MRGPRX2 Phosphorylation Assay Kit