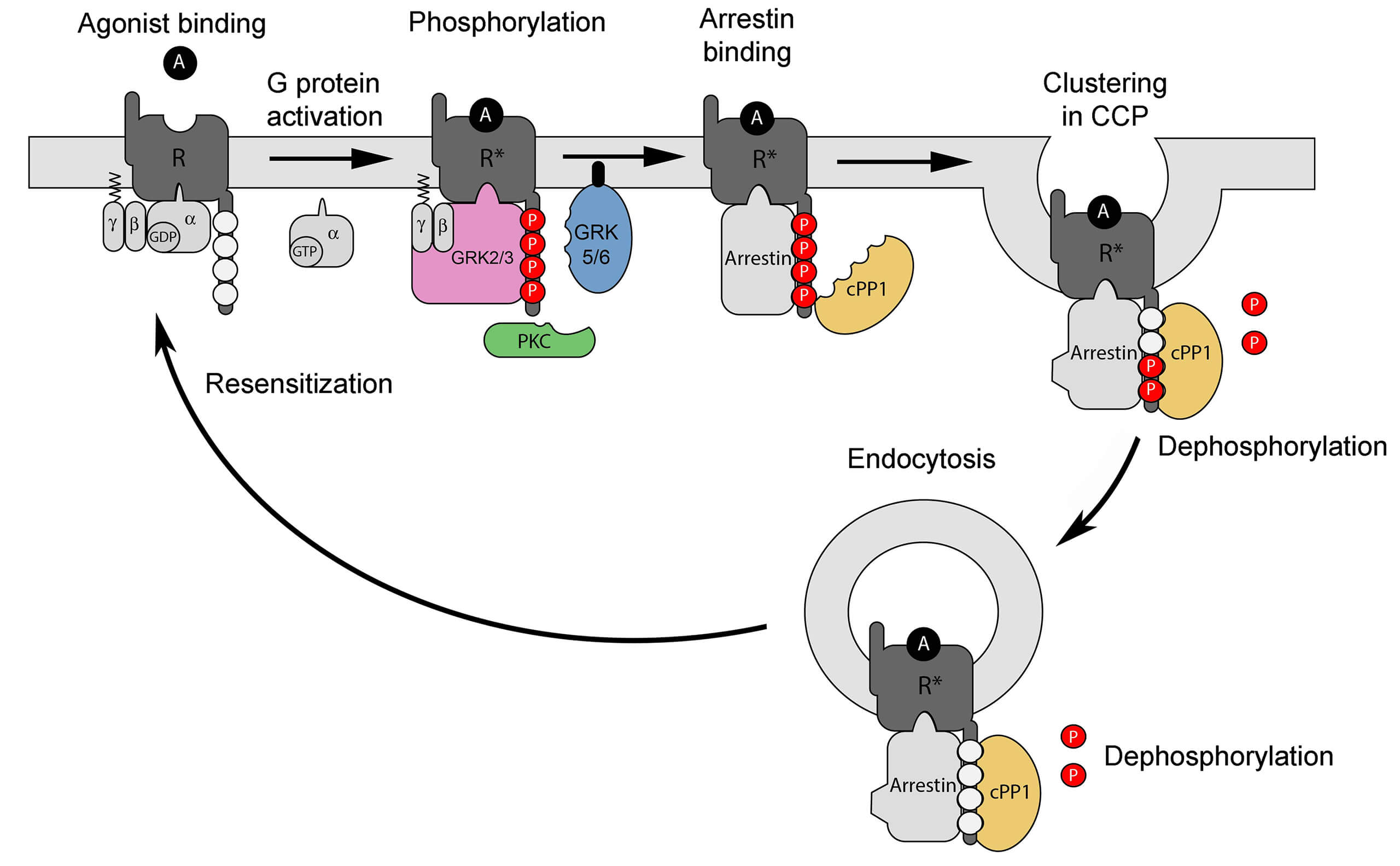
No results were found for the filter!
NEW
 pS327/pS329-CX3CR1 (phospho-CX3CR1 Chemokine...
pS327/pS329-CX3CR1 (phospho-CX3CR1 Chemokine... Serine327/Serine329 (S327/S329) is a major phosphorylation site of the CX3CR1 receptor. The pS327/pS329-CX3CR1 antibody detects phosphorylation in response to high- and low-efficacy agonists and after PKC activation. S327/S329...
$ 375.00 *
NEW
 pT363-CCR6 (phospho-Chemokine Receptor 6 Antibody)
pT363-CCR6 (phospho-Chemokine Receptor 6 Antibody) Threonine363 (T363) is a major phosphorylation site of the CCR6 receptor. The pT363-CCR6 antibody detects phosphorylation in response to high- and low-efficacy agonists and after PKC activation. T363 phosphorylation is a key regulator of...
$ 375.00 *
NEW
 pT360/pS361-CCR6 (phospho-Chemokine Receptor 6...
pT360/pS361-CCR6 (phospho-Chemokine Receptor 6... Threonine360/Serine361 (T360/S361) is a major phosphorylation site of the CCR6 receptor. The pT360/pS361-CCR6 antibody detects phosphorylation in response to high- and low-efficacy agonists and after PKC activation. T360/S361...
$ 375.00 *
NEW
 pS357-CCR6 (phospho-Chemokine Receptor 6 Antibody)
pS357-CCR6 (phospho-Chemokine Receptor 6 Antibody) Serine357 (S357) is a major phosphorylation site of the CCR6 receptor. The pS357-CCR6 antibody detects phosphorylation in response to high- and low-efficacy agonists and after PKC activation. S357 phosphorylation is a key regulator of...
$ 375.00 *
NEW
 pS326-CB2 (phospho-Cannabinoid Receptor 2...
pS326-CB2 (phospho-Cannabinoid Receptor 2... Serine326 (S326) is major phosphorylation site of the Cannabinoid Receptor 2 (CB2). The pS326-CB2 antibody detects phosphorylation in response to agonists. S326 phosphorylation is likely to be involved in efficient ligand sequestration...
$ 375.00 *
NEW
 pS351-APLNR (phospho-Apelin Receptor Antibody)
pS351-APLNR (phospho-Apelin Receptor Antibody) Serine351 (S351) is major phosphorylation site of the Apelin Receptor (APLNR). The pS351-APLNR antibody detects phosphorylation in response to agonists. S351 phosphorylation is likely to be involved in efficient ligand sequestration by...
$ 375.00 *
NEW
 pS319/pS322-H2 (phospho-H2 Histamine Receptor...
pS319/pS322-H2 (phospho-H2 Histamine Receptor... Serine319/Ser322 are major phosphorylation site of the H2 histamine receptor. The pS319/pS322-H2 antibody detects phosphorylation in response to high-efficacy agonists. S319/S322 phosphorylation is a key regulator of H2 desensitization,...
$ 375.00 *
NEW
 pS350-V2 (phospho-Vasopressin Receptor 2 Antibody)
pS350-V2 (phospho-Vasopressin Receptor 2 Antibody) Serine350 (S350) is major phosphorylation site of the Vasopresssin Receptor 2 (V2). The pS350-V2 antibody detects phosphorylation in response to agonists. S350 phosphorylation is likely to be involved in efficient ligand sequestration by...
$ 375.00 *
NEW
 pT359/pT360-V2 (phospho-Vasopressin Receptor 2...
pT359/pT360-V2 (phospho-Vasopressin Receptor 2... Threonine359/Threonine360 (T359/T360) is major phosphorylation site of the Vasopressin Receptor 2 (V2). The pT359/pT360-V2 antibody detects phosphorylation in response to agonists. T359/T360 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pS362/pS363/pS364-V2 (phospho-Vasopressin...
pS362/pS363/pS364-V2 (phospho-Vasopressin... Serine362/Serine363/Serine364 (S362/S363/S364) is major phosphorylation site of the Vasopresssin Receptor 2 (V2). The pS362/pS363/pS364-V2 antibody detects phosphorylation in response to agonists. S362/S363/S364 phosphorylation is likely...
$ 375.00 *
NEW
 pT378/pS380-V1A (phospho-Vasopressin Receptor...
pT378/pS380-V1A (phospho-Vasopressin Receptor... Threonine378/Serine380 (T378/S380) is major phosphorylation site of the Vasopressin Receptor 1A (V1A). The pT378/pS380-V1A antibody detects phosphorylation in response to agonists. T378/S380 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pT386/pS389-V1A (phospho-Vasopressin Receptor...
pT386/pS389-V1A (phospho-Vasopressin Receptor... Threonine386/Serine389 (T368/S389) is major phosphorylation site of the Vasopressin Receptor 1A (V1A). The pT386/pS389-V1A antibody detects phosphorylation in response to agonists. T386/S389 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pS397/pT398-V1A (phospho-Vasopressin Receptor...
pS397/pT398-V1A (phospho-Vasopressin Receptor... Serine397/Threonine398 (S397/T398) is major phosphorylation site of the Vasopressin Receptor 1A (V1A). The pS397/pT398-V1A antibody detects phosphorylation in response to agonists. S397/T398 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pS373/pS374-V1B (phospho-Vasopressin Receptor...
pS373/pS374-V1B (phospho-Vasopressin Receptor... Serine373/Serine374 (S373/S374) is major phosphorylation site of the Vasopressin Receptor 1B (V1B). The pS373/pS374-V1B antibody detects phosphorylation in response to agonists. S373/S374 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pT377/pT378-V1B (phospho-Vasopressin Receptor...
pT377/pT378-V1B (phospho-Vasopressin Receptor... Threonine377/Threonine378 (T377/T378) is major phosphorylation site of the Vasopressin Receptor 1B (V1B). The pT377/pT378-V1B antibody detects phosphorylation in response to agonists. T377/T378 phosphorylation is likely to be involved in...
$ 375.00 *
NEW
 pT271-M2 (phospho-M2 Muscarinic Acetylcholine...
pT271-M2 (phospho-M2 Muscarinic Acetylcholine... Threonine271 is a major phosphorylation site of the M2 Muscarinic Acetylcholine Receptor. The pT271-M2 antibody detects phosphorylation in response to high-efficacy agonists but not after PKC activation. T271 phosphorylation is a key...
$ 375.00 *
Recently viewed