Prices plus VAT plus shipping costs
Ready to ship today,
Delivery time appr. 5-8 days
- Order number: 7TM0319N
- Content: 100 µl
- Host: Rabbit
The non-phospho-µ-opioid receptor antibody is directed against the distal end of the carboxyl-terminal tail of mouse, rat and human MOP. It detects selectively the canonical form of MOP and none of the putative splice variants. It can be used to detect total MOP receptors in Western blots independent of phosphorylation. The non-phospho-MOP antibody can also be used to isolate and enrich µ-opioid receptors from brain lysates. It also detects MOP in cultured cells and tissue sections by immunohistochemistry. The non-phospho-MOP antibody has been validated using knockout mice (KO-Validated).
| Alternative Names | MOP, OPRM1, µ-Opioid Receptor, Mu Receptor |
| IUPHAR Target ID | 319 |
| UniProt ID | P35372 (human) P42866 (mouse) P33535 (rat) |
| Western Blot (WB) | 1:1000 |
| Immunocytochemistry (ICC) | 1:200 |
| Immunohistochemistry (IHC) | 1:100 |
| Species Reactivity | Human, Mouse, Rat |
| Host / Isotype | Rabbit / IgG |
| Class | Polyclonal |
| Immunogen | A synthetic peptide with the sequence LENLEAETAPLP which is present in carboxyl-terminal tail of human, mouse and rat MOP |
| Form | Liquid |
| Purification | Antigen affinity chromatography |
| Storage buffer | Dulbecco's PBS, pH 7.4, with 150 mM NaCl, 0.02% sodium azide |
| Storage conditions | short-term 4°C, long-term -20°C |
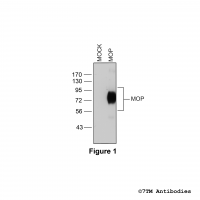
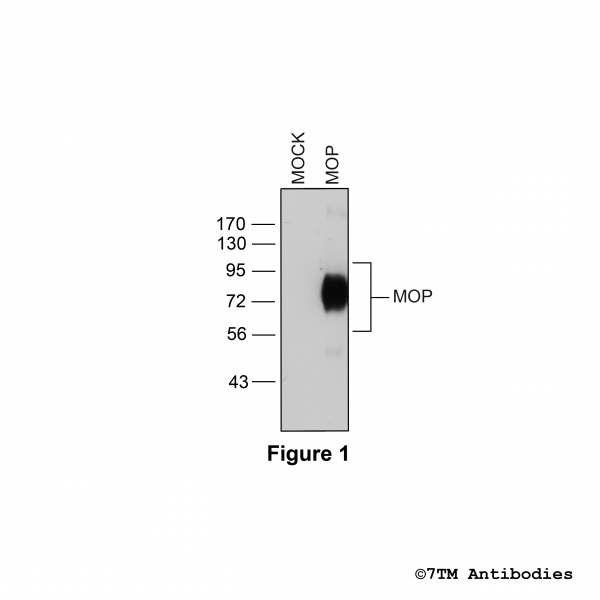
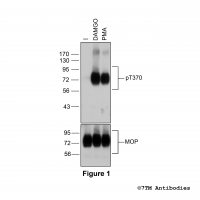
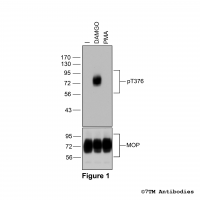
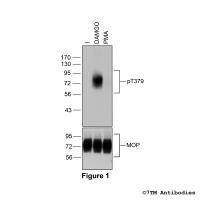
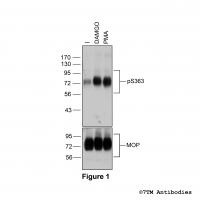
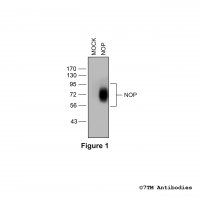
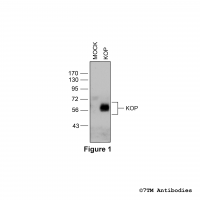
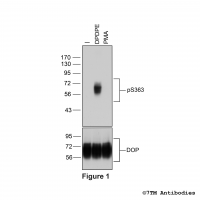
Figure 1. Validation of the µ-Opioid Receptor in transfected HEK293 cells. Native HEK293 cells (MOCK) or HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were lysed and immunoblotted with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000.
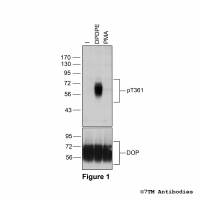
Figure 2. Immunocytochemical identification of µ-Opioid Receptor in HEK293 cells. HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were either not exposed or exposed to 10 μM DAMGO ([D-Ala2,N-MePhe4, Gly-ol]-enkephalin) for 30 min and immunocytochemically stained with anti-MOP (non-phospho-µ-opioid receptor) antibody (7TM0319N-WB) at a dilution of 1:200. Note, MOP receptors were confined to the plasma membrane in untreated cells (0 min). MOP receptors were seen predominantly in perinuclear clusters of vesicles after 30 min DAMGO exposure.
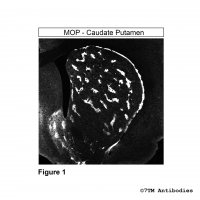
Figure 3. Immunohistochemical identification of µ-Opioid Receptor in caudate putamen. Sections were dewaxed, microwaved in citric acid, and incubated with anti-MOP (non-phospho-µ-Opioid Receptor) antibody (7TM0319N-WB) at a dilution of 1:100. Sections were then sequentially treated with biotinylated anti-rabbit IgG and avidin-biotin solution. Color was developed by incubation in 3-amino-9-ethylcarbazole (AEC), and sections were counterstained with hematoxylin. Note, MOP receptors were detected at the rim and as islets in the caudate putamen.
Figure 4. Immunohistochemical identification of µ-Opioid Receptor in spinal cord dorsal horn. Sections were dewaxed, microwaved in citric acid, and incubated with anti-MOP (non-phospho-µ-Opioid Receptor) antibody (7TM0319N-WB) at a dilution of 1:100. Sections were then sequentially treated with biotinylated anti-rabbit IgG and avidin-biotin solution. Color was developed by incubation in 3-amino-9-ethylcarbazole (AEC), and sections were counterstained with hematoxylin. Note, MOP receptors were uniformly detected at the plasma membrane of lung cells.
Figure 5. Immunohistochemical identification of µ-Opioid Receptor in dorsal root ganglia. Sections were dewaxed, microwaved in citric acid, and incubated with anti-MOP (non-phospho-µ-Opioid Receptor) antibody (7TM0319N-WB) at a dilution of 1:100. Sections were then sequentially treated with biotinylated anti-rabbit IgG and avidin-biotin solution. Color was developed by incubation in 3-amino-9-ethylcarbazole (AEC), and sections were counterstained with hematoxylin. Note, MOP receptors were detected predominantly in small- and medium-size cells.
Glück L, Loktev A, Moulédous L, Mollereau C, Law PY, Schulz S. Loss of morphine reward and dependence in mice lacking G protein-coupled receptor kinase 5. Biol Psychiatry. 2014 Nov 15;76(10):767-74. doi: 10.1016/j.biopsych.2014.01.021. Epub 2014 Feb 3. PubMed PMID: 24629717; PubMed Central PMCID: PMC4119866.
Illing S, Mann A, Schulz S. Heterologous regulation of agonist-independent μ-opioid receptor phosphorylation by protein kinase C. Br J Pharmacol. 2014 Mar;171(5):1330-40. doi: 10.1111/bph.12546. PubMed PMID: 24308893; PubMed Central PMCID: PMC3952808.
Just S, Illing S, Trester-Zedlitz M, Lau EK, Kotowski SJ, Miess E, Mann A, Doll C, Trinidad JC, Burlingame AL, von Zastrow M, Schulz S. Differentiation of opioid drug effects by hierarchical multi-site phosphorylation. Mol Pharmacol. 2013 Mar;83(3):633-9. doi: 10.1124/mol.112.082875. Epub 2012 Dec 13. PubMed PMID: 23239825; PubMed Central PMCID: PMC3583494.
Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. Deciphering µ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol. 2012 Nov;167(6):1259-70. doi: 10.1111/j.1476-5381.2012.02080.x. PubMed PMID: 22725608; PubMed Central PMCID: PMC3504992.
Grecksch G, Just S, Pierstorff C, Imhof AK, Glück L, Doll C, Lupp A, Becker A, Koch T, Stumm R, Höllt V, Schulz S. Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A μ-opioid receptor knock-in mice. J Neurosci. 2011 Sep 28;31(39):13890-6. doi: 10.1523/JNEUROSCI.2304-11.2011. PubMed PMID: 21957251; PubMed Central PMCID: PMC6633166.
Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011 Sep;164(2):298-307. doi: 10.1111/j.1476-5381.2011.01382.x. PubMed PMID: 21449911; PubMed Central PMCID: PMC3174411.